Fatty Acid Biosynthesis
The biosynthetic reaction pathway to a compound is usually not a simple opposite of its breakdown. In fatty acid synthesis, acetyl-CoA is the direct precursor only of the methyl end of the growing fatty acid chain. All the other carbons come from the acetyl group of acetyl-CoA but only after it is modified to provide the actual substrate for fatty acid synthase, malonyl-CoA.
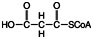
Malonyl-CoA contains a 3-carbon dicarboxylic acid, malonate, bound to Coenzyme A. Malonate is formed from acetyl-CoA by the addition of CO2 using the biotin cofactor of the enzyme acetyl-CoA carboxylase.
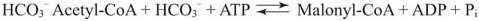
Formation of malonyl-CoA is the commitment step for fatty acid synthesis, because malonyl-CoA has no metabolic role other than serving as a precursor to fatty acids.
Fatty acid synthase (FAS) carries out the chain elongation steps of fatty acid biosynthesis. FAS is a large multienzyme complex. In mammals, FAS contains two subunits, each containing multiple enzyme activities. In bacteria and plants, individual proteins, which associate into a large complex, catalyze the individual steps of the synthesis scheme.
Initiation
Fatty acid synthesis starts with acetyl-CoA, and the chain grows from the “tail end” so that carbon 1 and the alpha-carbon of the complete fatty acid are added last. The first reaction is the transfer of the acetyl group to a pantothenate group of acyl carrier protein (ACP), a region of the large mammalian FAS protein. (The acyl carrier protein is a small, independent peptide in bacterial FAS, hence the name.) The pantothenate group of ACP is the same as is found on Coenzyme A, so the transfer requires no energy input:

In the preceding reaction, the S and SH refer to the thio group on the end of Coenzyme A or the pantothenate groups. The ~ is a reminder that the bond between the carbonyl carbon of the acetyl group and the thio group is a “high energy” bond (that is, the activated acetyl group is easily donated to an acceptor). The second reaction is another transfer, this time, from the pantothenate of the ACP to cysteine sulfhydral (–SH) group on FAS.
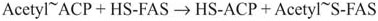
Elongation
The pantothenate –SH group is now ready to accept a malonyl group from malonyl-CoA:

Note that at this point, the FAS has two activated substrates, the acetyl group bound on the cysteine –SH and the malonyl group bound on the pantothenate –SH. Transfer of the 2-carbon acetyl unit from Acetyl~S-cysteine to malonyl-CoA has two features:
- Release of the CO2 group of malonyic acid that was originally put on by acetyl-CoA carboxylase
- Generation of a 4-carbon ß- keto acid derivative, bound to the pantothenate of the ACP protein
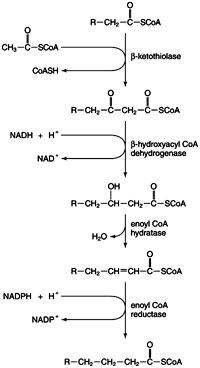
The ketoacid is now reduced to the methylene (CH2) state in a three-step reaction sequence.
1. Reduction by NADPH to form the ß- hydroxy acid derivative:
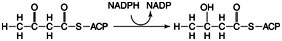
2. Dehydration, that is, the removal of water to make a trans-double bond:
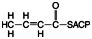
3. Reduction by NADPH to make the saturated fatty acid:
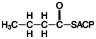
The elongated 4-carbon chain is now ready to accept a new 2-carbon unit from malonyl-CoA. The 2-carbon unit, which is added to the growing fatty acid chain, becomes carbons 1 and 2 of hexanoic acid (6-carbons).
Release
The cycle of transfer, elongation, reduction, dehydration, and reduction continues until palmitoyl-ACP is made. Then the thioesterase activity of the FAS complex releases the 16-carbon fatty acid palmitate from the FAS.
Note that fatty acid synthesis provides an extreme example of the phenomenon of metabolic channeling: neither free fatty acids with more than four carbons nor their CoA derivatives can directly participate in the synthesis of palmitate. Instead they must be broken down to acetyl-CoA and reincorporated into the fatty acid.
Fatty acids are generated cytoplasmically while acetyl-CoA is made in the mitochondrion by pyruvate dehydrogenase.This implies that a shuttle system must exist to get the acetyl-CoA or its equivalent out of the mitochondrion. The shuttle system operates in the following way: Acetyl-CoA is first converted to citrate by citrate synthase in the TCA-cycle reaction. Then citrate is transferred out of the mitochondrion by either of two carriers, driven by the electroosmotic gradient: either a citrate/phosphate antiport or a citrate/malate antiport as shown in Figure.
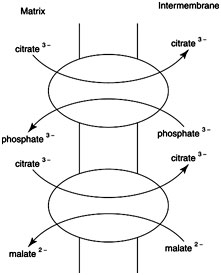
After it is in the cytosol, citrate is cleaved to its 2- and 4-carbon components by citrate lyase to make acetyl-CoA and oxaloacetate. Citrate lyase requires ATP.
Fatty acid biosynthesis (and most biosynthetic reactions) requires NADPH to supply the reducing equivalents. Oxaloacetate is used to generate NADPH for biosynthesis in a two-step sequence. The first step is the malate dehydrogenase reaction found in the TCA cycle. This reaction results in the formation of NAD from NADH (the NADH primarily comes from glycolysis). The malate formed is a substrate for the malic enzyme reaction, which makes pyruvate, CO2, and NADPH. Pyruvate is transported into the mitochondria where pyruvate carboxylase uses ATP energy to regenerate oxaloacetate.
Palmitate is the starting point for other fatty acids that use a set of related reactions to generate the modified chains and head groups of the lipid classes. Microsomal enzymes primarily catalyze these chain modifications. Desaturation uses O2 as the ultimate electron acceptor to introduce double bonds at the nine, six, and five positions of an acyl-CoA.
Elongation is similar to synthesis of palmitate because it uses malonyl-CoA as an intermediate.